Fęrsluflokkur: Vķsindi og fręši
19.12.2011 | 16:19
Opiš bréf til ķslenskra augnlękna - Mikilvęgi snemmtękra greininga į arfgengum augnsjśkdómum eykst
Flestir žeirra sem verša blindir eša alvarlega sjónskertir į unga aldri eša snemma į fulloršinsįrum verša žaš sökum arfgengra hrörnunarsjśkdóma ķ sjónhimnunni (RP og tengdir sjśkdómar). Engar mešferšir eru til viš žessum augnsjśkdómum ķ dag og žvķ er žaš hlutskipti žeirra sem eru meš žessa sjśkdóma aš tapa sķfellt meiri sjón meš įrunum og getur sjónmissirinn endaš ķ alblindu. Mjög misjafnt er hversu hratt žessir sjónhimnusjśkdómar žróast, allt frį įrum yfir ķ įratugi, og žvķ er žaš mjög einstaklingsbundiš hvenęr og hverjir verša blindir, sumir į unglingsįrum og ašrir seinna į fulloršinsįrum. Algengt er aš snemma ķ hrörnunarferlinu séu žessi sjśkdómar ekki vel greinanlegir nema sérstaklega sé skimaš eftir žeim. Žannig getur einstaklingur meš tiltekna birtingarmynd af slķkum sjśkdómi, sem gęti lżst sér ķ einungis 1/10 af fullu sjónsviši, nįttblindu en skarpri og mjög žröngri mišjusjón, stašist almennt sjónskerpupróf (lesa į stafaspjaldiš) sem oft eru lögš fyrir einstaklinga vegna endurnżjunar į ökuskķrteini. Viškomandi einstaklingur er hins vegar lögblindur.
Veršmęti sjónar
Dęmi er um žaš, bęši hérlendis og erlendis, aš einstaklingar sem eru meš žessa arfgengu hrörnunarsjśkdóma ķ sjónhimnu hafa żmist veriš aš fį enga greiningu, ranga greiningu eša greiningu sem kemur mjög seint ķ sjśkdómsferlinu, jafnvel žegar fólk er oršiš lögblint.
Blindrafélagiš hefur ķ tvķgang lįtiš Capacent Gallup gera fyrir sig skošanakannanir mešal almennings, 2009 og 2011, žar sem spurt er: Hvaš af tilteknum heilsufarįföllum fólk telji aš myndi hafa neikvęšustu įhrif į lķfsgęši sķn. Ašeins lömun skoraši hęrra ķ svörun heldur en sjónmissir. Žar fyrir nešan voru įföll eins og hjartaįfall, heilablóšfall, krabbamein og önnur įföll sem aušveldlega geta dregiš fólk til dauša. Śr žessu mį lesa įkvešiš veršmętamat og um leiš žį miklu angist sem fólk stendur frammi fyrir gagnvart žvķ aš missa sjónina. Žessar nišurstöšur gera rétta og snemmtęka greiningu augnlękna į augnsjśkdómum, hvort sem til eru viš žeim mešferšir eša ekki, mjög mikilvęga fyrir sįlarheill žeirra sjśklinga sem ķ hlut eiga og möguleika žeirra į aš njóta višeigandi ašstošar og žjónustu. Til dęmis meš žvķ aš vķsa žeim til Žjónustu og žekkingarmišstöšvar fyrir blinda, sjónskerta og daufblinda einstaklinga.
Žekkingu fleygir fram
Nś er žaš svo aš hrörnunarsjśkdómar ķ sjónhimnu eru fįséšir sjśkdómar. Tališ er aš nżgengi žeirra sé į bilinu 1/3000 – 1/3500. Žaš er žvķ ljóst aš žessir sjśkdómar eru ekki oft aš koma fyrir augu augnlękna.
Į undaförnum įrum hefur žekkingu į ešli žessara sjśkdóma fleygt fram og nś er svo komiš aš fjölmargar klķnķskar sjśklingatilraunir eru viš žaš aš fara ķ gang, žegar hafnar eša jafnvel komnar vel į veg. Um er aš ręša tilraunir meš genamešferšir, stofnfrumumešferšir, tilraunir meš rafeindasjón og lyfja- og taugafrumumešferšir, svo eitthvaš sé nefnt. Žegar fyrstu mešferširnar koma į almennan markaš, sem gęti veriš innan 5 įra, aš mati žeirra sem eru bjartsżnir, munu žeir sjśklingar standa best aš vķgi sem hafa rétta og nįkvęma greiningu. Żmislegt bendir einnig til žess aš mešal fyrstu og ašgengilegustu mešferšanna verši fyrirbyggjandi mešferšir sem muni stöšva eša hęgja allverulega į hrörnunarferlinu. Verši žaš raunin eykst mikilvęgi snemmtękrar og réttra greininga ennžį meira.
Aukin įrvekni
Um allan hinn vestręna heim kalla samtök žeirra sem eru meš žessa augnsjśkdóma nś eftir aukinni įrvekni augnlękna viš skimun og greiningu žessara arfgengu hrörnunarsjśkdóma ķ sjónhimnu. Meš réttri og snemmtękri greiningu aukast möguleikar sjśklinga į žvķ aš geta notiš réttrar mešferša žegar žęr vonandi fara aš lķta dagsins ljós į nęstu 5 – 10 įrum.
Žar sem engin von var gefin fyrir 15 – 20 įrum er nśna von.
Kristinn Halldór Einarsson
Formašur Blindrafélagsins,
samtaka blindra og sjónskertra į Ķslandi
15.6.2009 | 10:03
Til hamingju Einar
Morgunblašiš segir frį žvķ ķ frétt aš Einar Stefįnsson prófessor viš lęknadeild Hįskóla Ķslands, hafi veriš sęmdur gullmedalķu evrópsku augnlęknaakademķunar og jafnframt veriš tekinn inn ķ akademķuna. Full įstęša er til aš óska Einar til hamingju meš žessa višurkenningu.
Žessi frétt vekur athygli į žvķ aš hversu mikiš er ķ gangi į sviši rannsókna ķ augnvķsindum og hef ég reynt aš gera hluta af žvķ skil hér į žessari sķšu. Ešli mįlsins samkvęmt žį eru allar žessar rannsóknir aš fįst viš aš leita lękninga viš augnsjśkdómum sem valda óafturkręfum sjónmissi. Ķ seinustu bloggfęrslu minn birti ég framvindu skżrslu yfir rannsóknir sem gerš var grein fyrir į įrsfundi AVRO(The Association for Research in Vision and Ophthalmology,) sem haldinn var ķ Fort Lauderdale ķ Bandarķkjunum 1 - 7 maķ sl. Skżrsla žessi var sérstakeiga tekin saman fyrir Retina International, sem hafa undanfarin 30 įr beitt sér fyrir rannsóknum į žessu sviši. Žaš er rétt aš hafa ķ huga aš žęr rannsóknir sem nś eru ķ gangi eru tķmafrekar og mörg įr geta lišiš žar til ķ ljós kemur hvort aš žęr skila almennum mešferšum.

|
Einar Stefįnsson heišrašur |
| Tilkynna um óvišeigandi tengingu viš frétt | |
14.6.2009 | 14:17
Augnsjśkdómar - Framvinduskżrsla augnrannsókna frį įrsfundi AVRO 2009
Hér mį lesa skżrslu (į ensku) frį rįšstefnu og įrsfundi AVRO (The Association for Research in Vision and Ophthalmology,) sem haldinn var ķ Fort Lauderdale ķ Bandarķkjunum 1 - 7 maķ sl. Ķ žessari skżrslu mį lesa aš rannsóknir sem nś eru ķ gangi gefa vonir um aš mešferšir gagnvart ólęknandi augnsjśkdómum, sem valda alvarlegri sjónskeršingu eša jafnvel blindu, eru aš fęrast nęr. Žessar nišurstöšur eru afrakstur margra įra og įratuga grunnrannsókna og žekkingaröflunar. Žessi skżrsla var sérstaklega tekin saman fyrir Retina Intarnational. Af gefnu tilefni vil ég hvetja žį sem eru ķ samskiptum viš augnlękna hér į landi vegna augnsjśkdóma, aš bera skżrsluna undir sinn augnlękni, ef til umręšu er hvort mögulegar mešferšir muni verša til ķ framtķšinni og hvort eitthvaš af žvķ sem fjallaš er um ķ skżrslunni geti hugsanlega komiš aš gagni. Varšandi žį sem eru meš RP vil ég sérstaklega benda į umfjöllunina um RetinaComplex bętiefniš.
Retinal Disease: Progress Reports from the Twelfth Annual Vision Conference and the ARVO 2009 Annual Meeting
Prepared by Elaine A. Richman, Ph.D., Richman Associates, LLC, USA
The reports below were prepared exclusively for Retina International. They are based on presentations made by scientists and clinicians at the Twelfth Annual Vision Conference (“Mechanisms of Macular Degeneration”) and the ARVO 2009 Annual Meeting, in Ft. Lauderdale, Florida, held consecutively from May 1 – 7, 2009. The range of topics included artificial vision, genetic influences on disease processes, interventions to control disease, retinal cell imaging, and countless others related to possible causes, treatments, and cures for retinal degenerative diseases. Many possible explanations are emerging. Some may turn out to be relevant to one condition and not another, or in one animal model and not another. Regardless, the many possible explanations reflect progress in understanding retinal degenerative conditions.
More clues to rod and cones survival in RP
Connie Cepko from Harvard Medical College and her colleagues are working to understand the relationship between rod and cone photoreceptor death in retinitis pigmentosa (RP). Typically, a gene variant in rods causes the rod cells to die. But why, then, do cones also succumb? The researchers’;; work with mice suggests that part of the answer might relate to a protein called HDAC4: reduction in HDAC4 during normal retinal development causes rods to die; in a mouse model of retinal degeneration, supplementation with HDAC4 extends rod and cone cell survival. Related to cone death, they found a possible relationship between cone death and decreased activity of an insulin signaling pathway and perhaps nutrient depletion. Giving insulin to mice with retinal degenerative disease provided a degree of cone survival. Removing insulin accelerated cone demise. Ultimately, these findings could lead to development of therapies for intervening at various stages of RP.
Is cone death in RP caused by “starvation”?
Claudio Punzo of Harvard Medical School says that cone death in retinitis pigmentosa (RP) could be caused by starvation and explains why he believes this is so, related to the observations about insulin mentioned above. He and colleagues studied four types of mice with gene mutations that cause rod photoreceptor cell death. The researchers looked at subsequent onset of cone death and found it associated with the insulin/TOR pathway, a regulator of cell growth. Conversely, when they provided cones with additional insulin, the cones survived. Hence, their thinking that cone cells in RP starve to death. (Insulin is needed to transport the nutrient glucose into cells.) They further suggest that the rod death leads to a perturbation in the cone-RPE relationship. The RPE (retinal pigment epithelium) is the source of nutrients to the photoreceptor cells. In all four types of mice, the cones began to die when approximately the same density of rods was observed. Punzo does not suggest insulin treatment for RP; rather it was used in this study to bolster the starvation theory of cone involvement in RP.
Oxidative damage as another variable in rod and cone death in RP
Rods in the retina use a lot of oxygen. When they die in RP, the amount of oxygen in other portions of the retina goes up and causes oxidative damage to cones. It would make sense, therefore, that treatment with anti-oxidants would spare the cones. The logic of this approach is what Peter Campochiaro of Johns Hopkins University described in pigs and in mouse models of RP. The researchers are examining various drugs for inhibiting oxidative damage. They are also looking at gene therapies to improve patients’;; own anti-oxidant resources for prolonging cone survival. A combination of drug and gene therapy might be the most effective.
A role for a growth factor, too, in cone death in RP
Researchers have identified a protein that increases cone photoreceptor survival in rodent models of retinitis pigmentosa. The protein is a growth factor known as rod-derived cone viability factor (RdCVF). It appears to be involved in the defense against oxidative stress. When the growth factor was used to treat rats with autosomal dominant RP, their retinas showed not only more cone cell survival but also an unusual amplitude in retinal electrical activity. RdCVF was delivered by injecting the protein intraocularly or by delivering the protein-making gene using an adeno-associated virus delivery system. Jose-Alain Sahel of INSERM and his colleagues are looking at how this viability factor is tied to oxidative stress and to the function and survival of cone photoreceptors.
Retinal progenitor cells to replace photoreceptor cells
Research continues into ways to replace photoreceptor cells using retinal progenitor cells in retinitis pigmentosa and other degenerative retinal diseases where vision loss is due to photoreceptor cell death. It has been shown in animal studies that transplanted retinal progenitor cells (RPCs) from fetal, newborn, or adult animals are capable of migrating throughout the degenerating retina, becoming nerve cells, integrating into the retina and circuitry of the central nervous system, and even responding to light. The maturation and survival of RPCs appears to be dependent on numerous intrinsic and extrinsic factors. Also important are the source of the donor cells, their stage of development, their exposure to and regulation by various cellular and extracellular agents including those of the immune system, and the way these cells are delivered to the retina (e.g., by injection into the vitreous or subretinal space; on a synthetic scaffolding placed subretinally). Retinal transplant researcher Henry Klassen of the University of California at Irvine reminded the audience at ARVO 2009 that many other questions need answering before retinal cell transplant can become a viable therapy for replacing photoreceptor cells. The answers will require a multidisciplinary approach with experts not only from molecular biology but also from tissue engineering and other fields..
Norman Radtke and colleagues recently reported potentially encouraging findings from a Phase 2 clinical studies where they transplanted neural retinal progenitor cell layers, with fetal retinal pigment epithelium attached, into the subretinal space beneath the macula of patients with RP. Some enhanced vision was reported, although other researchers including Marco Zarbin of the New Jersey Medical School suggest that the results may be explained by a diffusion effect where growth factors from the implanted tissues dispersed to surrounding viable tissue and enhanced the response rather than by replacement of lost photoreceptors.
Overriding a nonsense mutation for treating Usher syndrome 1c: commercial aminoglycosides and PTC124
At ARVO 2009, researchers from the Technion-Israel Institute of Technology and the University of Mainz in Germany described experimental compounds for treating the USH1C gene nonsense mutation that causes USH1, the most severe form of Usher syndrome. A nonsense mutation causes a halt to the production of the protein that a gene is normally programmed to make. The researchers investigated several compounds—commercial aminoglycosides (e.g. G418, paromomycin), modified aminoglycosides (NB30, NB54), and the novel compound PTC124—that show promise for treating other genetic disorders (e.g., cystic fibrosis, muscular dystrophy). The compounds work by inducing a read-through of the gene. In cultured cells and mouse retina with the Usher gene mutation, the researchers found that the commercial aminoglycosides and PTC124 successfully induced a read-through and restored protein expression and function in a dose dependent manner. Much more work is necessary to understand the toxicity and efficacy of these compounds.
High density parafoveal rings as possible endpoints in RP clinical trials
In drug trials for retinitis pigmentosa, to show efficacy, the U.S. Food and Drug Administration requires tests of visual field sensitivity and best corrected visual acuity. Researchers are looking at other clinical tests that might be even more sensitive to changes in disease progression. Alan Bird and his colleagues from London’;;s Moorfields Eye Hospital and UCL Institute of Ophthalmology found that the density of parafoveal rings, determined by fundus autofluorescence and correlated with pattern electroretinogram, indicate an area where cone function and the outer retinal layer, as indicated by optical coherence tomography, are preserved. As RP progresses, the rings grew progressively smaller. These parafoveal rings may be an additional endpoint for the FDA to consider in clinical trials of therapies for RP.
RP, pregnancy, and breast feeding
Italian researcher Luca Iacobelli, of the Italian Institute of Neurotrauma, and colleagues reported at ARVO 2009 on the effect of pregnancy and breast-feeding on visual acuity in women with retinitis pigmentosa. The only statistically significant finding occurred when best corrected visual acuity at month one of pregnancy and at five months post-partum was compared. All the women were breast feeding and the researchers suggest a possible relationship between decreased visual acuity and depletion of vitamin A and docosahexaenoic acid (DHA) through the mother’;;s milk. Post- partum vitamin supplementation is advised.
Nanoparticle delivery of genes in Leber’;;s congenital amaurosis
Researchers, including Muna Naash, from the University of Oklahoma recently demonstrated the successful use of RPE65 nanoparticles (plasmids expressing human RPE65 cDNA compacted into rod-like particles with a lysine peptide conjugated to polyethylene glycol) for delivering a gene therapy for treatment of Leber’;;s congenital amaurosis, a retinal degenerative disorder caused by mutations on the RPE65 gene. Other researchers have succeeded in delivering versions of the RPE65 gene using a viral vector, most notably to eyes of Briard dogs carrying the RPE65 mutation. The nanoparticle approach has the potential to overcome limitations of viral vectors including random integration into the host genome, inflammation, and more severe outcomes including death. It also demonstrates superiority over other non-viral gene delivery approaches (e.g., liposomal delivery, electroporation of naked DNA) in its transfection ability and duration of expression, say the researchers. The findings indicate that successful and sustained gene expression and functional improvement can be attained using the nanoparticle approach.
Promising results from Sirion for treatment of geographic atrophy
Sirion Therapeutics, Inc., recently announced interim results from its Phase 2 clinical trial of their drug fenretinide for the treatment of geographic atrophy (GA) associated with dry age-related macular degeneration. The positive results put the drug on a fast-track with the U.S. Food and Drug Administration. “The benefits of Fast Track include scheduled meetings to seek FDA input into development plans, the option of submitting a New Drug Application in sections rather than all components simultaneously, and the option of requesting evaluation of studies using surrogate endpoints.” (http://www.accessdata.fda.gov/scripts/cder/onctools/Accel.cfm#FastTrack) Fenretinide, provided in pill form, is a vitamin A binding protein antagonist and may work by reducing the formation or effect of drusen under the retinal pigment epithelium. Patients with lesions of all sizes who received 300 milligrams of fenretinide daily had slower rates of growth of their geographic atrophy than patients in control groups. A Phase 3 trial with many more than the 245 subjects already studied is being planned.
The “artificial retina” for stimulating vision lost to RP
Although photoreceptors cells die in RP, other neuronal cells of the retina remain capable of transmitting signals that reach the brain to be interpreted as vision. These cells, mainly retinal ganglion cells, if stimulated with enough electrodes in a fashion that is coordinated with light and shapes in the visual field, can provide patients with functional vision, or so researchers are hoping. Work is being done by several groups to develop a retinal implant that will electrically control these cells. Among these groups is one supported by the U.S. Department of Energy Office of Science. It is called the Artificial Retina Project and is under the leadership of Mark Humayun of the Doheny Eye Institute in Los Angeles. Another, called Retinal Implant AG, is led by Eberhart Zrenner of University Eye Hospital Tübingen.
The first retinal implant is attached to the surface of the retina (epiretinal). The other, by Zrenner’;;s group, is subretinal.
With the epiretinal implant a stimulus in the visual field is captured by a tiny camera mounted on eyeglasses worn by the patient. The information is sent to a belt-worn microprocessor where it is converted to an electronic signal and transmitted to the retinal implant in a fashion that mimics patterns in the visual field. The stimulation sent to each electrode is independent of the signal sent to the other electrodes. The patterns of stimulation “seen” by the brain correspond to the spatial and temporal stimulation of the electrodes in the retinal implant. The researchers are testing a new 60-electrode unit and comparing the results to earlier tests of an implant consisting of 16 electrodes. Twenty-one people with advanced retinitis pigmentosa (little or no light perception), in the U.S., Mexico, Great Britain, France, and Switzerland, have been fitted with the newer unit.
A just released report describing the outcome in a patient with the 16-electrode epiretinal implant show that the device enabled him to detect patterns of stimulation (i.e., demonstrated when rows of electrodes at right angles were stimulated one row at a time; patient drew lines indicating pattern). Furthermore, when he was asked to view a video monitor showing high-contrast square-wave gratings, he could detect distances between lines that corresponded to the spacing between neighboring electrodes. All patients with the implants, the researchers reported, detected phosphenes when the electrodes are stimulated.
The sub-retinal implant, in contrast to the epiretinal implant, essentially replaces the photoreceptor cell layer with an implant having stimulus electrodes on its surface. There is no external camera or computer; all electronic components are placed under the retina in a 3 x 3 x0.1 mm chip, except a small power supply, connected by a thin cable, ending behind the ear. The researchers reported on an advanced transchoroidal technique for implanting their newest device, which consists of a 1500-electrode chip that is stimulated by light and 4 x 4 electrodes for direct stimulation. Despite the complexity of implanting the subretinal microelectrode array, no damage appears to occur to retinal tissue even in patients implanted up to three months. Patients report seeing a whitish round dot when individual electrodes are stimulated directly. When direct stimulation is presented in a pattern meant to represent a certain letter, some patients are able to correctly name the letter. By means of the light sensitive chip the most recent three patients were able to to discern the direction of fine stripes. One patient was able to read letters 4 to 8 cm in size presented on a table in regular reading distance under dim illumination, and combine them to words and recognize and precisely localize unknown objects such as a banana or an apple.
Many questions concerning electronic epiretinal or subretinal devices have yet to be answered with regard to device fabrication, packaging, and location, surgical technique for implantation, long-term tissue tolerance to the devices and to the unique electrical stimulation, characterization of ganglion cell activity, and much more. Basic, preclinical, and clinical studies continue in the hands of scientists involved with the Artificial Retina Project and Retinal Implant AG and also with others including Optobionics with its Artificial Silicon Retina (ASR) implant, IMI Intelligent Medical Implants AG with its Learning Retinal Implant System, and the EPI RET3 epiretinal array.
Ganglion cells engineered to respond to light successfully trigger a response in the visual cortex
Changing the behavior of one type of neuron in the central nervous system to take over the function of another type of cell is a huge challenge. Each cell has its own innate regulatory system and responsibility, which was considered immutable until recently. But the potential for such a toggle therapy for retinal degenerative diseases is huge. John Flannery of University of California, Berkeley, and his colleagues have found that they can confer light sensitivity (the typical venue of rod and cone photoreceptor cells) on retinal ganglion cells in cell culture, zebrafish larvae, and now mice. Using a viral vector they delivered an engineered light sensitive protein (the glutamate receptor, LiGluR) by intravitreal injection to retinal ganglion cells in a mouse model of retinal degeneration (rd-1 mice). They then stimulated the ganglion cells with light, studied the response in the brain’;;s primary visual cortex (V1), and found a robust response and light-evoked responses at all post-injection time points. The researchers conclude that LiGluR is worthy of study as a possible therapy for restoring light sensitivity in patients who are blind because of photoreceptor loss.
Algorithm predicts which RPE65 mutation causes pathogenicity
Ed Stone of the University of Iowa and his colleagues are looking at ways to differentiate disease-causing mutations from nonpathogenic gene polymorphisms to help patients and families with retinal disorders understand their risk and to help scientists plan clinical trials and explain varied outcomes in their research. They have developed an algorithm that calculates an "estimate of pathogenic probability." It is based on the prevalence of a specific variation, its segregation within families, and its predicted effects on protein structure. Using the algorithm to evaluate 11 missense variations in the RPE65 gene of patients with Leber congenital amaurosis they were able to predict eight mutations as disease-causing variants. The algorithm may be useful to evaluate the pathogenicity of missense variations in other disease genes.
Warning of possible damage from fundus autofluorescence imaging of the RPE
Fundus autofluorescence (FAF) imaging is one of the newer techniques for examining the retina in health and disease. It identifies lipofuscin in retinal pigment epithelial cells and appears to provide information that cannot be gotten from other imaging techniques like fundus photography, fluorescein angiography, or optical coherence tomography. David Williams of the University of Rochester and his colleagues are finding that it may have a downside, however: possible damage to the retina from the blue light used in FAF imaging.
Lipofuscin derives mainly from uptake by RPE cells of photoreceptor degradation products. It fluoresces yellow when it is stimulated with light in the blue range. Lipofuscin accumulates with age and in certain retinal degenerative diseases. FAF imaging can be used to assess normal lipofuscin accumulation and also disease status in conditions such as Stargardt disease, Best’;;s vitelliform macular dystrophy, and age-related macular degeneration.
But when Dr. Williams’;; group examined the potential in monkeys for light-induced retinal damage from FAF , they found a deleterious effect at levels of light exposure that were even lower than the maximum permissible levels (MPE) set by the American National Standards Institute (ANSI). The fact that other clinical procedures such as slit lamp examination, fundus photography, fluorescein angiography, and retinal surgery are often performed at light levels close to the MPE makes the study potentially relevant across all of ophthalmology. Patients who have retinal disease, which may make them particularly vulnerable to blue light, should discuss with their physicians the importance of minimizing light exposure during retinal exams.
Gene Replacement Clinical Trials
UPenn Trial on LCA- RPE65 Gene Therapy – Dr. A. Cideciyan, University of Pennsylvania, Philadelphia, PA USA
Preclinical studies on gene replacement therapy in animal models of Leber Congenital Amaurosis (LCA) were very successful. Specifically, the RPE65 gene was replaced in these cases using an AAV viral vector with excellent safety and efficacy that has restored a measure of sight in animals for a number of years. Based on this work, a Phase I clinical trial began in 2007 in LCA subjects with the RPE65 mutation with the approval of the FDA and support of the National Institutes of Health. The trial is led by Dr. Samuel Jacobson at the University of Pennsylvania in Philadelphia where the patients are first entered into the trial and examined before and after surgery. Surgery and gene-vector production occurs at the University of Florida under the leadership of Drs. William Hauswirth and Barry Bryne.
Dr. Artur Cideciyan gave an update on this trial. The primary outcome of the trial is safety; secondary outcomes include changes in vision. The trial was initially planned for 3 cohorts (groups) of patients, each with three individual subjects, The first cohort consisted of 3 young adults between 21 and 24 years of age. These patients received subretinal injections of the AAV2 vector with the normal RPE65 gene. The results available through the first 90 days post-treatment have been published and were encouraging. In terms of safety, there were no vector-related Serious Adverse Events (SAEs) and no systemic toxicities. All patients self-reported and increase in visual sensitivity in their treated study eye compared with their control eye. This was especially noticeable under reduced ambient light conditions. Using a full-field stimulus test under dark-adapted conditions, the study eye showed significant sensitivity increases.
Specialized methods of vision testing corroborated that there were significant improvements in sensitivity localized to the area of treatment in the injected eye. The gene therapy procedure improved both day vision originating in cone photoreceptors as well as night vision originating in rod photoreceptors of the LCA patients. Day vision could be improved up to 50-fold and night vision up to 63,000-fold compared to pretreatment levels.
Dr. Cideciyan stated that the first cohort of patients has passed the 1-year time point and continue to do well. Specifically, there are no vector-related serious adverse events and the intervention appears to be safe. Based on the positive results in the first cohort, the Data Safety and Monitoring Committee has allowed the study to continue to the second cohort which includes adults injected with a higher dose of vector and to the third cohort which includes children.
CHOP Trial on LCA- RPE65 Gene Therapy
Dr. J. Bennett, University of Pennsylvania, Philadelphia, PA USA
Dr. Bennet reported that at the Children’;;s Hospital of Philadelphia (CHOP) as well as in Naples, Italy and Ghent, Belgium, work continues on investigating the effects of RPE65 gene replacement in LCA subjects.
The first subject received a single unilateral subretinal injection of AAV2.hRPE65v2 in October of 2007 and, since that time, 10 additional individuals have been enrolled and received injections. Baseline testing, surgery and follow-up testing are carried out at CHOP. CLIA testing for the RPE65 mutation was done by Dr. E. Stone at the Univ. of Iowa.
The first 3 subjects enrolled (Naples, Italy) are young adults (19-26 yrs at the time of injection) and are now 15 months post-injection. They continue to do well as to both safety and efficacy. Duplicate baseline testing is now done at CHOP and in Naples serving to generate a robust data set.
Individuals enrolled over the last year include 4 children. All have recovered visual function with improvement in their nystagmus. They are reading without aids.
The upper age limit (72 yr) was eliminated in the protocol since a therapeutic effect was observed in the 26 yr old individuals in the first cohort. The oldest individual enrolled to date is 44 yr old.
All the juvenile and adult subjects are doing well. Two of these patients are from Ghent, Belgium so a third follow-up site is planned for this location.
In conclusion, Dr. Bennett said that the program in on target and the patients are happy with the results – all asking to have their second eye injected.
RetinaComplex Clinical Study in Retinitis Pigmentosa Patients
Drs. Javier Romero and Theo van Veen, University CEU Cardenal Herrera and Mediterranean Ophthalmology Foundation, Valencia, Spain and University of Tuebingen, Tuebingen, Germany
Previous work by Prof. van Veen established the efficacy of using a combination of potent antioxidants called RetinaComplex in slowing the progression of retinal degeneration in animal models of RP. Please see the minutes from last year’;;s SMAB meeting for a position paper by Retina International on safety and efficacy issues in such use.
In the absence of Dr. Romero, Professor van Veen described the current clinical study. This is a hospital-based prospective, randomized double-blind clinical study with an appropriate control group being performed in Valencia, Spain. The first 12 months of the study have now been concluded with a total of 44 subjects with Retinitis Pigmentosa. 23 subjects received RetinaComplex and 21 subjects received placebo for 12 months. Written consent was obtained from the participants after they were given a reasonable explanation of the study details. A complete history of each participant with respect to age, gender, clinical symptoms, etc. was collected using a questionnaire.
Blood samples are collected to check for 1) glycosylated hemoglobin (a marker for metabolic control and possible diabetes) 2) zinc and vitamin E (to check for possible patient self-supplementation) and 3) malondialdehyde (a lipid peroxidation product that is a marker for oxidative stress).The fundus of each participant was exampled with an ophthalmoscope and macular OCT performed. Miltifocal ERG as well as other functional studies such as automated perimetry was performed and a general health evaluation questionnaire was obtained. All these baseline parameters are being compared with the same parameters obtained after 12 month RetinaComplex intake using appropriate statistical analysis.
From the intermediate data collected so far, no difference could be established between paired data, except for the amplitude of the multifocal erg. The placebo group showed a statistical difference between the data collected at the beginning of the study and the end of the first 12 month period. Of importance though, the patients receiving RetinaComplex showed no statistically significant difference between the two sets of data. This confirmsthat there is a slower progression of disease in the treated subjects compared with those getting only placebo. Although this study was initially planned for only 1 year, the promising results obtained makes it worthwhile to continue the study for a longer period if proper funding can be obtained. The results of the first two years of the study will be available in autumn of this year.
Neuroprotection Clinical Trials
CNTF-ECT Clinical Trials – Ms. Kathleen Dickinson – Neurotech, Lincoln, RI USA
CNTF is a natural compound found in the body that helps to protect neuronal cells from damage, hence is a “neuron-survival” and “neurotrophic” agent. It has been shown to slow the course of retinal degeneration in a number of RD animal models. A problem with neurotrophic agents though is in the method of delivery to the retina. However, Neurotech has devised an ingenious capsule that is implanted within the vitreous cavity of the eye that delivers a sustained and safe dose of CNTF to the retina. This is called “Encapsulated Cell Technology” or NT-501-ECT. Preclinical experiments were successful using this device, showing relative safety as well as efficacy. Phase I of an FDA-approved trial also has successfully been completed.
Dr. Dickinson first apologized that Dr. Weng Tao, who was initially scheduled to give the Neurotech update was, at the last minute, unable to attend. She then proceeded to inform the group as to the current advanced-phase trials on subjects with RP and on those with Geographic Atrophy (GA). The trials are as follows: CNTF2 for dry AMD/GA; CNTF3 for late stage RP; CNTF4 for early stage RP. For example, 51 patients with dry AMD are being studied in the GA trial. To date in this ongoing work, the safety results are good in that Neurotech has encountered no significant treatment-related Serious Adverse Events (SAEs). No serum antibodies have developed against CNTF or the encapsulated cells that produce it. Dr. Dickinson reported that the biological effect of NT-501 has been identified. OCT images have demonstrated that there is improved definition of the Outer Nuclear Layer (ONL) of the retina in treated subjects at 12 months, compared to baseline images. A significant increase in retinal thickness, measured as total macular volume was determined by OCT. This was a dose dependent effect and observed in all three CNTF protocols. These results are consistent with published reports from preclinical observations. Retinal thickness was determined by the Duke Reading Center to not to be due to cystoid macular edema, epiretinal membrane formation, vitreoretinal traction or choroidal neovascularization.
Thus, the results appear to be encouraging such that CNTF-ECT might be the first treatment generally available to both RP and dry AMD patients.
Vķsindi og fręši | Breytt 15.6.2009 kl. 10:04 | Slóš | Facebook | Athugasemdir (0)
28.5.2009 | 15:59
Śr fundargerš įrsfundar vķsinda og rįšgjafarnefndar Retina International
Žann maķ 4 var haldinn įrsfundur Scientific and Medical Advisisory Board of Retina International ķ Fort Lauderdal ķ Kalifornķu. Į fundinn voru męttir margir af fremstu augnlęknum og vķsindamönnum ķ fręšunum ķ dag.
Mešal žess sem mį finna ķ fundargeršini er umfjöllun um RetinaComplex bętiefniš. Ķ žeirri umfjöllun segi m.a:.
„From the intermediate data collected so far, no difference could be established between paired data, except for the amplitude of the multifocal erg. The placebo group showed a statistical difference between the data collected at the beginning of the study and the end of the first 12 month period. Of importance though, the patients receiving RetinaComplex showed no statistically significant difference between the two sets of data. This confirms that there is a slower progression of disease in the treated subjects compared with those getting only placebo. Although this study was initially planned for only 1 year, the promising results obtained makes it worthwhile to continue the study for a longer period if proper funding can be obtained. The results of the first two years of the study will be available in autumn of this year."
Ķ fundargeršinni er einnig fjallaš um klķnķskar lyfjatilraunir bandarķska fyrirtękisins Neurotech. Ķ nišurlagi žeirrar umfjöllunar segir:
„Thus, the results appear to be encouraging such that CNTF-ECT might be the first treatment generally available to both RP and dry AMD patients"
Vķsindi og fręši | Breytt 14.6.2009 kl. 13:44 | Slóš | Facebook | Athugasemdir (0)
11.10.2008 | 14:43
Ķslenskur augnlęknir žįtttakandi ķ merkilegri tilraun
Margir af žeim sem eru meš RP augnsjśkdóminn, sem er arfgengur sjónhimnu hrörnunarsjśkdómur, hafa ķ nokkurn tķma vitaš um störf Ragnheišar Bragadóttur ķ Noregi. Ragnheišur er įn vafa fremst mešal ķslenskra vķsindamanna žegar kemur aš rannsóknum į RP. Mikill fengur vęri af žvķ aš fį hana til landsins til aš halda fyrirlestur um žęr rannsóknir sem hśn er žįtttakandi ķ. Žaš er rétt aš hafa ķ huga aš sś ašferšarfręši sem žessar rannsóknir byggja į, ž.e. aš nota veirur til aš smita stökkbreyttar frumur meš heilbrigšu geni, er eitthvaš sem mun geta nżst į mun fleiri svišum en ķ augnlękningum. Rannsóknin, sem er undir stjórn Robin Ali į Morfield sjśkrahśsinu žykir žaš merkileg aš margar greinar hafa birtar um hana ķ virtustu lęknatķmaritum ķ heiminum. Meš žvķ aš smella hér mį lesa ein af žessum greinum.
Ķ jślķ sķšastišnum gerši ég grein fyrir nišurstöšum frį rįšstefnu Retina International ķ Helsinki, en žar var umrędd rannsókn sérstaklega kynnt og er hśn ein af įstęšum žess aš meiri bjartsżni er nś rķkjandi mešal bęši vķsinda og leikamanna aš mešferšir og lękningar į įšur ólęknandi augnsjśkdómum séu ķ sjónmįli, į kanski nęstu 10 - 15 įrum. Umfjöllunina mį sjį hér, en ķ henn er jafnframt gerš grein fyrir öšrum rannsóknum or tilraunum sem eru ķ gangi.

|
Fį hluta sjónar į nż |
| Tilkynna um óvišeigandi tengingu viš frétt | |
Vķsindi og fręši | Breytt s.d. kl. 15:30 | Slóš | Facebook | Athugasemdir (1)
Bandarķska fyrirtękiš Neurotech hefur fengiš leyfi bandarķskra yfirvalda (FDA) fyrir byltingakenndum tilraunir į sjśklingum til mešferšar į RP og ellihrörnun ķ augnbotnum (Dry AMD). Ellihrörnun ķ augnbotnum er algengasta örsök blindu į Ķslandi og ķ hinum vestręna heimi. Mešferšarśrręši gagnvart RP hafa ekki veriš til stašar og mjög takmörkuš gagnvart AMD. Vonast er eftir žvķ aš fyrstu nišurstöšur žessara tilraun lķti dagsins ljós nęsta vor.
Ķ grein sem ég skrifaši į bloggsķšuna mķna ķ jślķ s.l. gerši ég ķ stuttu mįli grein fyrir žessum tilraunum. Sjį hér.
Hér fer fréttatilkynning frį Neurotech.
Neurotech Granted Fast Track Designations from the FDA for NT-501 in Two Indications?Retinitis Pigmentosa and Dry Age-Related Macular Degeneration
Lincoln, RI (September 3, 2008) - Neurotech Pharmaceuticals, Inc., a privately-held biotechnology company focused on the development of sight-saving therapeutics for chronic retinal diseases, announced today that the U.S. Food and Drug Administration (FDA) has granted Fast Track designations for NT-501 for the treatment of visual loss in two indications? retinitis pigmentosa (RP) and the dry form of age-related macular degeneration (dry AMD). NT-501 is an intraocular, cell containing polymer implant designed to provide continuous, long-term release of the therapeutic protein Ciliary Neurotrophic Factor (CNTF) directly into the back of the eye by means of the Company's proprietary Encapsulated Cell Technology. CNTF, a well established neurotrophic factor, rescues dying retinal photoreceptors and protects them from degeneration.
"We remain on track to announce top line results from our two Phase 2/3 studies in RP and our Phase 2 study in dry AMD by early 2009," said Ted Danse, President and CEO of Neurotech. "The receipt of Fast Track designations for NT-501 in these indications is an important component in our ongoing product development strategy, allowing us to potentially accelerate our two clinical development programs as we seek to provide much needed treatment options for patients facing these devastating diseases."
The Fast Track program, established under the FDA Modernization Act of 1997, provides for expedited regulatory review of a drug that demonstrates the potential to address an unmet medical need for the treatment of serious or life-threatening conditions. Under the program, the FDA will take such actions as are appropriate to expedite the development and review of the application for approval of such product, including potentially accepting, on a rolling basis, portions of the marketing application for review prior to the completion of the final registration package.
About Neurotech's Clinical Programs
Phase 2/3 Studies in Retinitis Pigmentosa (RP)
Neurotech is conducting two Phase 2/3 trials of NT-501 for the treatment of visual loss associated with RP-one consisting of patients with earlier stage disease (60 patients) and the second consisting of patients with later stage disease (60 patients). Both trials are randomized, multi-centered, double-masked, sham-controlled dose ranging studies. Each patient receives either a high or low dose NT-501 implant in one eye an a sham treatment in the fellow eye. The primary efficacy endpoint is visual field sensitivity for the early-stage RP study and best corrected visual acuity for the late-stage RP study.
Phase 2 Study - Dry Age-related Macular Degeneration (Dry AMD)
This randomized, multi-centered, double-masked, sham-controlled study is evaluating NT-501 in 48 subjects with an advanced stage of dry AMD called geographic atrophy. Each subject receives either a high or low dose NT-501 implant or a sham treatment in one eye only. Best corrected visual acuity is the primary efficacy endpoint of this study.
About the Diseases
Retinitis Pigmentosa (RP) is an inherited disease that causes the retina's rod and cone photoreceptors to gradually degenerate leading to loss of vision and blindness. The symptoms of RP predominately appear in young adults and affect approximately 100,000 people in the United States and over 1 million people worldwide. At this time there is no known cure or effective treatment for RP.
Age-related macular degeneration (AMD) is a chronic progressive disease of the macula that results in the loss of central vision. It is the leading cause of blindness in elderly people in the developed world. There are two forms of AMD?dry and wet. Dry AMD is the most common form of AMD representing approximately 90% of all AMD cases. In its later stages dry AMD can lead to the degeneration of photoreceptors and retinal pigment epithelial cells, a chronic condition called geographic atrophy (GA). GA affects approximately 1 million people in the United States for which there currently are no effective treatments.
About Encapsulated Cell Technology
Neurotech's core technology platform is Encapsulated Cell Technology (ECT), a unique technology that allows for the long term, sustained delivery of therapeutic factors to the back of the eye. ECT implants consist of cells that have been genetically modified to produce a specific therapeutic protein and are encapsulated in a semi-permeable hollow fiber membrane. The diffusive characteristics of the hollow fiber membrane are designed to promote long-term cell survival by allowing the influx of oxygen and nutrients while simultaneously preventing direct contact of the encapsulated cells with the cellular and molecular elements of the immune system. The cells continuously produce the therapeutic protein which diffuses out of the implant at the target site. ECT therefore enables the controlled, continuous delivery of therapeutic factors directly to the retina, bypassing the blood-retina barrier.
About Neurotech Pharmaceuticals, Inc.
Neurotech is developing sight-saving therapeutics for the treatment of chronic retinal diseases. The Company's lead product candidate, NT-501, is currently in late-stage clinical development for retinitis pigmentosa (RP) and dry age-related macular degeneration (dry AMD). RP is the leading inherited cause of blindness for which there is no current treatment. The Company's portfolio of product candidates also includes treatments for wet AMD and diabetic macular edema. All of Neurotech's development programs are based on the Company's proprietary Encapsulated Cell Technology (ECT). ECT uniquely enables the controlled, continuous delivery of biologics directly to the back of the eye, overcoming a major obstacle in the treatment of retinal disease.
MEDIA CONTACT:
Rich Small
Vice President, CFO
Neurotech Pharmacuticals, Inc.
r.small@neurotechusa.com
http://neurotechusa.com/
Vķsindi og fręši | Breytt s.d. kl. 19:05 | Slóš | Facebook | Athugasemdir (0)
3.9.2008 | 18:52
Einar Stefįnsson fęr veršlaun į sviši augnrannsókna

|
Einar Stefįnsson fęr veršlaun į sviši augnlękninga |
| Tilkynna um óvišeigandi tengingu viš frétt | |
Vķsindi og fręši | Breytt s.d. kl. 20:23 | Slóš | Facebook | Athugasemdir (0)
25.7.2008 | 16:56
Vinna andoxunarefni gegn arfgengri hrörnun ķ sjónhimnu?
Vķsindamenn eru farnir aš hvetja žį sem eru meš RP aš taka inn įkvešna samsetningu andoxunarefna, žvķ lķkur séu į žvķ aš žaš komi aš gagni viš aš hęgja į žróun hrörnunarferlisins įn žess aš hafa ķ för meš sér alvarlegar aukaverkanir.
Į rįšstefnu Retina International ķ Helsinki hitti ég konu frį S-Afrķku sem sagši frį žvķ aš ķ hennar tilviki hefši hrörnunarferliš stöšvast frį žeim tķma žegar hśn hóf inntöku žessara andoxunarefna. Męlingar augnlęknis hefšu stašfest žetta.
Žessi andoxunarefni eru nś fįanleg og heita RetinaComplex.
Į fundi vķsindanefndar Retina International (SMAB) sem var haldinn ķ aprķl sķšast lišnum var samžykkt yfirlżsing varšandi notkun andoxunarefna gegn RP.
Hér fer lauslegri žżšingu į yfirlżsingunni, fyrir nešan er enska śtgįfan:
"Óhįšar nišurstöšur frį vel virtum rannsóknarašilum eru samdóma um aš įkvešin blanda af andoxunarefnum séu įrangursrķk til aš hęgja į žróun hrörnunar ķ sjónhimnu ķ tilraunadżrum. Jįkvęša višbrögš ķ öllum žessum tilraunadżrum gefa til kynna aš notkun andoxunarefna geti gefiš góša raun sem mešferš viš RP og öšrum tengdum sjśkdómum
Öryggisatriši mešferšarinnar ķ dżratilraununum hafa sżnt sig vera ķ lagi, auk žess sem efnin sem eru notuš eru žekkt fyrir aš vera örugg fyrir menn og eru ekki efni sem eru flokkuš sem lyf.
Retina International hlakkar til aš sjį nišurstöšur klķnķskrar rannsóknar į mönnum, um notkun andoxunarefna til aš hęgja į hrörnunarferli ķ sjónhimnu, sem nś eru ķ gangi ķ Mediterranean Ophthalmology Foundation, Valencia, Spain (Prof. F.J. Romero)".
At the Annual Retina International Scientific and Medical Advisory Board [SMAB] meeting held at the ARVO congress in April the board issued the following statement on the use of anti oxidants for RP:
"Independent evidence from well respected laboratories agrees that combinations of antioxidant supplements are successful in slowing retinal degeneration in RD animal models. Positive effects in all these animal models may indicate that such treatment could be effective in most or all forms of RP and allied diseases irrespective of molecular diagnosis.
Safety seems assured from the animal testing done to date and from the fact that the supplements used are known to be well tolerated in humans and are not controlled substances.
Retina International looks forward to the results of human clinical studies for this promising treatment to slow the progression of Retinal Degeneration. These studies are ongoing at the Mediterranean Ophthalmology Foundation, Valencia, Spain (Prof. F.J. Romero)".
Vķsindi og fręši | Breytt 28.7.2008 kl. 13:15 | Slóš | Facebook | Athugasemdir (0)
21.7.2008 | 17:24
Mešferšir viš arfgengum hrörnunarsjśkdómum ķ sjónhimnu ķ nśtķš og framtķš - Žrišji hluti
Hér er žrišji og seinasti hluti erindis Gerald J. Cahder (Ph.D.,M.D.hc - Chief Scientific officer, Doheny Retina International USC Medical school ķ Los Angeles) į rįšstefnu Retina International ķ Helsinki 4 og 5 jślķ, žar sem hann dró saman helstu nišurstöšur śr žeim erindum sem flutt voru į rįšstefnunni.
Ég bišst velviršingar ef žżšingar eru ónįkvęmar į einhverjum stöšum og biš um aš viljinn sé tekinn fyrir verkiš. Žessari žżšingu er ekki ętlaš aš vera lęršur pistill heldur fyrst og fremst til upplżsinga fyrir žį sem eru meš RP og ašra tengda sjśkdóma og fjölskyldur žeirra og vini.
Hér fer lausleg žżšing į žrišja og seinasta hluta įvarpsins:
"Hvaša klķnsku rannsóknum mį bśast viš ķ nįinni framtķš į sjaldgęfari sjśkdómum
Nokkrir rannsóknarhópar vinna nś aš žvķ aš koma ķ gang tilraunum į nokkrum af sjaldgęfari sjśkdómum.
- Leber congenital amaurosis (LCA):Žrķr rannsóknarhópar hafa hafiš klķnķskar tilraunir ķ genamešferš žar sem göllušu geni er skipt śt fyrir heilbrigt gen ķ sjśklingum meš įkvešna tegund af LCA. Aš minnsta kosti einn hópur til višbótar er einnig meš klķnķska rannsókn ķ undirbśningi.
- Stargard: Dr. Rando Allikmets er įsamt samstarfsmönnum sķnum aš gera tilraun meš aš skipta śt stökkbreyttu geni sem veldur Stargad fyrir heilbrigt gen ķ nagdżrum. Fram til žessa eru hafa eingöngu brįšabyrgša nišurstöšur fengist, en žęr lofa góšu. Ef tilraunin reynist vera örugg og įrangursrķk žį mun undirbśningur undir klķnķskar rannsóknir hefjast.
Fleiri rannsóknarhópar eru ķ svipušum sporum. - Usher sjśkdómar: Rannsóknarhópar vinna nś aš genamešferš į Usher1 og Usher3 meš tilraundżrum.
Vandamįl hefur veriš aš fį góš tilraunadżr, žaš vandmįl hefur nś veriš leyst.
Dr. Flannery hefur skżrt frį žvķ aš įrangur er aš nįst. - Choroideremia:Vķsindamenn eru nįlęgt žvķ aš fį nagdżr til aš nota sem tilraundżr meš choroideremia sjśkdóminn. Genamešferš meš tilraundżrin er ķ undirbśningi. Ef žęr reynast įrangursrķkar žį getur undirbśningur undir klķnķskrar tilraunir meš sjśklinga hafist.
- Samsvarandi tilraunir eru ķ gangi varšandi ašra sjaldgęfa sjśkdóma svo sem eins og Retinoschisis.
Hvaš meš ellihrörnun ķ augnbotnum (AMD)?
Nśverandi AMD mešferšir
Žurr AMD- Bętiefnamešferš. Andoxunarefnin sem voru rannsökuš ķ klķnķskri rannsókn AREDAS eru nś fįanleg.
Vot AMD - Nokkur lyf hafa veriš samžykkt af viškomandi yfirvöldum ķ žó nokkrum löndum, sem er ętlaš aš hęgja į nżmyndun afbrigšilegra ęša. Best žekkta lyfiš er Lucentis.
Bętiefni og ellihrörnun ķ augnbotnum (Dry AMD)
AREDAS tilraunin meš andoxunarefni
- National Eye Institude (USA) hefur lokiš viš tilraun žar sem AMD er mešhöndlaš meš bętiefnum (andoxunarefnum). Tilraunin var nefnd AREDS (Age-related Eye disase study)
- Tilraunin sżndi fram į aš aš įkvešin bętiefni voru hjįlpleg gagnvart AMD. Andoxunarefnin sem voru rannsökuš voru: B-carontene, og C-vķtamķn, E-vķtamķn įsamt zinki.
- Žessi andoxunarefni nįšu ašeins aš hęgja į ferlinu į įkvešnu stigi sjśkdómsferilsins, ž.e. um mišbik.
- Žessi andxunarefni eru komin ķ sölu en rétt er aš leita rįša hjį lękni įšur en inntaka hefst.
Klķniskar mešferšir - vot ellihrörnun ķ augnbotnum (Wet AMD)
- Lyfiš Lucentis virkar į vota ellihrörnun ķ augnbotnum. Lyfiš virkar gegn VEGF, próteini sem veldur myndun nżrra afbrigšilegra ęša ķ augnbotnum. Lyfiš bętir sjón.
- Eitt af vandamįlunum viš Lucentis er aš žaš žarf aš sprauta žvķ inn ķ augun.
- Annaš vandamįl er aš žaš žarf aš endurtaka mešferšin aftur og aftur.
- Žrišja vandamįliš er sķšan hversu dżrt lyfiš er. Annaš svipaš lyf, Avastin, er fįnlegra į mun lęgra verši.
AMD klķnķskar tilraunir - Klķnķskar genatilraunir
- Fyrirtękiš GeneVec er meš ķ gangi klķnķska tilraun meš genamešferš gegn votri ellihrörnun ķ augnbotnum. Ķ tilrauninni er PEDF geninu komiš fyrir ķ auganu. Fyrsta stigi tilraunarinnar er lokiš og voru öryggisnišurstöšur mjög góšar.
- PEDF próteiniš er nįttśrulegt sem nżst getur bęši gegn AMD og RP.
- Ef nišurstöšur tilraunarinnar verša jįkvęšar žį eru lķkur į aš nota megi ašferšina gegn žurri ellihrörnun ķ augnbotnum (Dry AMD) og RP įsamt votri ellihrörnun ķ augnbotnum (Wet AMD).
Klķnķskar rannsóknir į votri ellihrörnun ķ augnbotnum
Nokkur önnur efni sem hamlaš geta gegn nżmyndun afbrigšilegra ęša ķ augnbotnum. Sem dęmi mį nefna:
- Fyrirtękiš Oxigene er meš efniš Combretastatin, sem nżtt hefur veriš gegn nżęšamyndun ķ krabbameinsmešferš.
- Žetta efni er nś ķ klķnķskri tilraun gegn votri ellihrörnun ķ augnbotnum.
- Efni mį gefa ķ ęš og žvķ nokkuš öruggt.
- Önnur sambęrileg efni eru ķ žróun.
AMD klķnķskar rannsóknir - Bętiefni
- Bętiefnatilraun sem kallast AREDS2 meš Lutein er nś ķ gangi į nokkrum AMD sjśklingum undir stjórn National Eye Institution (USA).
- Lutein/Zeaxanthin er efni sem unniš er śr litarefnum įvaxta og gręnmetis og virkar sem andoxunarefni og er ętlaš aš verja ljósnemanna fyrir skemmdum af völdum sśrefnis.
- Žetta er rannsóknir sem mun taka mörg įra aš ljśka.
- Žangaš til er rétt aš fara aš rįšum mömmu og borša įvexti og gręnmeti.
Nišurstöšur
Žó nokkrar klķnķskar tilraunir į hinum mismunandi hrörnunarsjśkdómum ķ augum eru nś ķ gangi, eša viš žaš aš hefjast.
Til dęmis žį hafa genamešferšir ķ dżrum meš RP sżnt mjög jįkvęšar nišurstöšur, ekki eingöngu aš sjón hafi nįšst til baka heldur voru langtķmaįhrifin mjög jįkvęš. Er hér komin lękning?
Grunnrannsóknir sem snśa aš genamešferš, lyfjamešferš, bętiefnum og rafeindasjón eru mjög lofandi og nokkrar klķnķskar tilraunir eru žegar hafnar.
Rafeindasjón gęti veriš besti möguleiki žeirra sem eru blindir eša mjög alvarlega sjónskertir til aš öšlast aftur sjón.
Aš lokum
- Ķ dag getum viš mešhöndlaš og jafnvel lęknaš tilraunadżr meš margs konar hrörnunar sjśkdóma ķ sjónhimnu.
- Margar klķnķskar rannsóknir eru aš hefjast žannig aš mešferšir geta veriš framundan.
- Allt er žetta mjög dżrt og tekur langan tķma....... en hver treystir sér til aš setja veršmiša į aš fį aftur glataša sjón."
Vķsindi og fręši | Breytt 28.7.2008 kl. 13:16 | Slóš | Facebook | Athugasemdir (3)
17.7.2008 | 16:57
Mešferšir viš arfgengum hrörnunarsjśkdómum ķ sjónhimnu ķ nśtķš og framtķš - Annar hluti
Hér er annar hluti erindis Gerald J. Cahder (Ph.D.,M.D.hc - Chief Scientific officer, Doheny Retina International USC Medical school ķ Los Angeles) į rįšstefnu Retina International ķ Helsinki 4 og 5 jślķ, žar sem hann dró saman helstu nišurstöšur śr žeim erindum sem flutt voru į rįšstefnunni.
Ég bišst velviršingar ef žżšingar eru ónįkvęmar į einhverjum stöšum og biš um aš viljinn sé tekinn fyrir verkiš. Žessari žżšingu er ekki ętlaš aš vera lęršur pistill heldur fyrst og fremst til upplżsinga fyrir žį sem eru meš RP og ašra tengda sjśkdóma og fjölskyldur žeirra og vini.
Hér fer lausleg žżšing į öšrum hluta įvarpsins. Įvarpiš mun verša sett fram ķ žremur hlutum.
"Klinkskar tilraunir ķ nśtķš og framtķš
Nś skulum viš beina athygli okkar aš tveimur mešferšarśrręšum žegar fįir eša engir ljósnemar eru eftir į lķfi.
Möguleg mešferšarśrręši ķ slķkum tilvikum gętu falist ķ:
- Ķgręšsla ljósnema eša stofnfruma.
- Notkun rafeindasjónar.
Klķnķskar tilraunir meš ķgręšslu (transplantation) ljósnema
- Lengi hefur veriš unniš aš žeirri hugmyndin hvort hęgt vęri aš skipta śt daušum ljósnemum fyrir nżja ljósnema.
- Lķtil klķnķsk rannsókn į mönnum er nś ķ gangi meš ķgręšslu ljósnema. Rannsóknin er undir stjórn Dr. Norman Radtke ķ Bandarķkjunum.
- Tilraunin hefur sżnt aš ašferšin viršist vera örugg, en žvķ mišur žį hafa nišurstöšur ekki sżnt aš ašferšin hafi bętt sjón hjį einstaklingum sem neinu nemur.
Meš vķsan til žess aš eingöngu lķtill, eša enginn įrangur hefur nįšst ķ aš bęta sjón meš žessum tilraunum, hjį bęši mönnum og dżrum, er erfitt aš sjį aš žessi tilraun muni leiša til įrangurrķkrar mešferšar ķ framtķšinni.
Ķgręšsla stofnfruma (Transplantation: Stem Cells)
Rannsóknir į stofnfrumum og innsetningu žeirra er nżtt og mjög spennandi sviš.
- Stofnfrumur er frumu sem hafa žann eiginleika aš geta fjölgaš sér og breyst ķ nęstum žvķ hvernig frumur sem er ķ lķkamanum.
- Fręšilega séš mętti žvķ setja stofnfrumur ķ sjónhimnuna žar sem eru daušir ljósnemar og stofnfrumurnar ęttu sķšan aš geta žróast ķ ljósnema og leyst af hólmi žį sem eru daušir.
- Hinsvegar žarf aš gefa stofnfrumunum įvešin lķffręšileg merki žannig aš aš žęr žróist ķ aš vera virkir ljósnemar sem koma aš gagni, frekar en aš žróast ķ einhverjar ašrar frumur.
Klķnķskar tilraunir meš stofnfrumur
- Fréttir hafa borist af žvķ aš tilraun žar sem stofnfrumur voru notašar ķ tilraun meš sjśkling hafi veriš stöšvašar ķ Bandarķkjunum. Fyrirtękiš sem ķ hlut į hefur hins vegar ekki stašfest aš stofnfrumur hafi veriš notašar.
- Fréttir hafa borist um aš stofnfrumumešferšir hafi veriš stundašar ķ einhverjum rķkjum. Litlar upplżsingar er hinsvegar aš hafa um žessar mešferšir hvaš varšar öryggi og virkni.
- Mun meiri rannsóknarvinna žarf aš eiga sér staš įšur en stofnfrummešferš getur oršiš raunhęft mešferšarśrręši viš RP.
Rafeindasjón ķ staš glatašrar sjónar
Hér er um tvo flokka aš ręša:
- Ķgręšslu rafeindaörflaga į heilabörk.
- Ķgręšslu rafeindaörflaga ķ sjónhimnuna -fyrir framan eša aftan sjónhimnuna.
Ķ tilvikum ķgręšslu ķ sjónhimnu, žį eru margskonar nįlganir og hannanir ķ gangi hjį hinum żmsu hópum vķša ķ heiminum.
Ķgręšsla rafeindaörflaga į heilabörk
Žrķr rannsóknahópar eru, eša hafa unniš, viš aš hanna rafeindasjón sem mišar aš žvķ fara algerlega framhjį augunum.
- Einn hópurinn hefur žegar framkvęmt ótķmabęrar ķgręšslu ķ menn meš litlum įrangri.
- Annar hópurinn einbeitir sér ašallega aš grunn rannsóknum.
- Žrišji hópurinn hefur nįš mjög góšum įrangri meš ķgręšslur ķ apa og klķnķskar rannsóknir eru ķ undirbśningi.
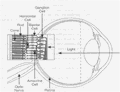
Hvernig virkar rafeindasjón?
- Bśnašurinn notast viš rafstraum til aš fara framhjį göllušum eša daušum ljósnemum til aš örva ašra hluta (nonphotorecptorcell) sjónhimnunnar.
- Myndir koma frį utanįliggjandi myndvél sem komiš er fyrir bak viš geraugu sjśklings.
- Myndirnar eru fluttar ķ gegnum tölvu til örflaga (eloctrodes called array) sem hafa veriš gręddar į sjónhimnuna til aš endurskapa myndina sem send er til heilans.
Tilraunir meš rafeindasjón
Hópar ķ mörgum löndum vinna aš žvķ aš žróa rafeindasjón
Fjórir hópar vķšs vegar um heiminn hafa žegar sett mismunandi hannašan rafeindasjónbśnaš ķ menn.
- Optobionics Co. (Chicago, USA) - Slök hönnun žannig aš bśnašurinn virkar illa eša alls ekki.
- Tvö mjög góš žżsk fyrirtęki - Fyrsta stig tilraun meš menn ķ gangi - Retina Implant AG.
- Second Sight (Sylmar, Kalifornķa USA) - Undir stjórn Dr. M.S. Humayun, hefur ķgręšsla įtt sér staš ķ 6 sjśklinga į fyrsta stigi klķnķskrar tilraunar og meira en 10 sjśklingar hafa fengiš ķgręšslu į öšru stigi tilraunarinnar sem nżlega fór ķ gang.
Fyrsta stigs tilraunir į sjśklingum - Hvaš hefur gerst
Samfelldar tilraunir hafa įtt sér staš frį įrinu 2002 į mönnum žar sem notast hefur veriš viš 16 örflögu bśnaš.
- Ķgręšsla hefur įtti sér staš hjį sex sjśklingum. Ekkert af bśnašinum bilaši.
- Allir sjśklingarnir nįšu aš sjį ašgreinda hluti (phosphenes) og gįtu framkvęmt sjónręn verkefni ķ rżmi og hreyfingu.
- Hreyfanleiki og ferlihęfni batnaši.
Rafeindasjón - Framtķšin
- Nokkrar klķnķskar tilraunir hafa veriš settar ķ gang. Ef žęr reynast įrangursrķkar er lķklegt aš tęki til almennra nota verši fįanleg ķ nįnustu framtķš.
- Hönnun og tękni hefur fleygt fram ķ žvķ aš fjölga örflögum sem gręša mį į sjónhimnuna.
- Fręšilega žį er ljóst aš žaš žarf 1000 örflögur til aš skapa sjón nógu skrapa til lestrar og til aš geta žekkt mannsandlit.
- Rafeindasjónin er kannski besti möguleiki, žeirra sem eru alvarlega sjónskertir eša blindir af völdum RP eša žurr AMD, til aš öšlast sjón į nż.
Aš lokum fyrir RP...
Nokkrar klķnķskar tilraunir eru ķ undirbśningi. Ašferšarfręši fyrir nokkrar ašrar tegundir mešferšar hafa veriš sannprófašar.
- Til dęmis, genamešferš į hundi, RP-LCA, sżndi ekki eingöngu aš sjón sem var töpuš vannst til baka, heldur voru langtķmaįhrifin mjög jįkvęš.
- Ašrar grunnrannsóknir, į svišum svo sem eins og genamešferš, stofnfrumurannsóknum, lyfjamešferš, bętiefnum og rafeindaörflögu innsetningu eru mjög lofandi fyrir klķniskar tilraunir ķ nśtķš og framtķš."
Ķ žrišja hluta mun verša fjallaš um sjaldgęfa augnsjśkdóma og ellihrörnunarsjśkdóma (AMD).
Vķsindi og fręši | Breytt 28.7.2008 kl. 13:14 | Slóš | Facebook | Athugasemdir (0)

 Gerald J. Cahder Helsinki 3hl.
Gerald J. Cahder Helsinki 3hl.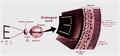

 einarlee
einarlee
 sjos
sjos
 gislihelgason
gislihelgason
 thjalfi
thjalfi
 gummigisla
gummigisla
 gunnimar
gunnimar
 hrannarb
hrannarb
 nonniblogg
nonniblogg
 kristinnjon
kristinnjon
 andmenning
andmenning
 retinita
retinita
 meistarinn
meistarinn
 leifurl
leifurl
 sjonni-boy
sjonni-boy




